How To Draw Pop Fizz Step By Step
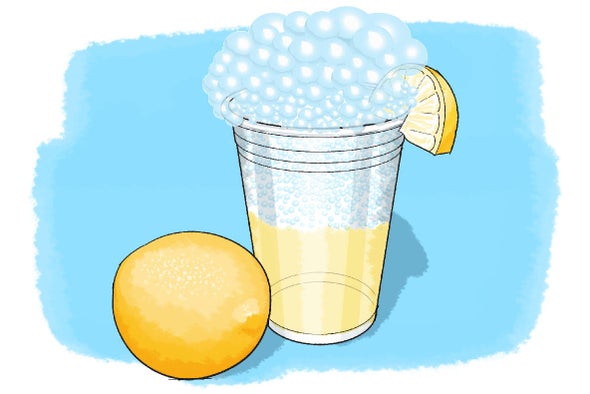
Make Your Own Fizzy Lemonade
A refreshing science activity from Science Buddies
Key concepts
Chemistry
Acids
Bases
Chemical reactions
Introduction
Did you know there are thousands of different types of soda around the world? Do you think you would like cheese-flavored soda? What about octopus-flavored soda? Although flavors might differ from country to country, one thing all sodas have in common is the bubbles! Soda isn't soda without that fizz—but have you ever wondered how they create those bubbles? In this activity you will make your own bubbly drink while exploring the reactions that create that famous fizz.
Background
Acids and bases are all around us; in our dishwashing liquid, our medicines—even our foods! They're extremely useful in part because they react so strongly to each other. We can use these reactions to help us clean, ease a sick stomach or make food taste more interesting.
A base is a compound that can donate negatively charged hydroxide ions. When you add a base to water the basic compound will break apart, and the addition of its hydroxide ions makes the solution more basic. Bases are found in many household cleaning supplies as well as in medicine to help neutralize stomach acid for people who suffer from heartburn.
An acid, in contrast, is a compound that can donate a positively charged hydrogen ion (or proton). When you add an acid to water it will come apart, making the solution more acidic with the addition of its hydrogen ions. Although some acids can be dangerous, we also need them to survive. For instance, as we chew, the acid in saliva helps break down food for digestion!
When you combine an acid and a base you initiate an acid–base reaction. When there are an equal number of hydrogen and hydroxide ions present the acid and base will neutralize each other, forming a salt and water. In this activity you will explore the reaction that takes place when you combine an acid with a basic carbonate.
Materials
- Cold water
- Two lemons
- Teaspoon of sugar (more or less to taste)
- Teaspoon of baking soda
- Plastic or glass cup
- Stirring spoon
- Lemon juicer/press (recommended)
- Knife (for slicing lemons)
- Adult helper
- Ice (optional)
Preparation
- Have your adult helper cut both lemons in half.
Procedure
- Use the lemon juicer to squeeze all of the juice from each lemon into your glass. Taste a few drops of the juice. How would you describe the taste?
- Add an equal amount of cold water to the lemon juice in your glass. Taste the mixture again. Does it taste different than just the lemon juice? In what way?
- Carefully add one teaspoon of baking soda. Use your spoon to stir the mixture. What happens when you add baking soda to the lemon juice? What do you see and hear happening in the glass?
- Use your spoon to stir one teaspoon of sugar into the mixture. Add ice if you want it cold!
- Taste your concoction! What do you notice about the taste? How does it differ from the lemon juice alone?
- Extra: Allow your mixture to sit for an hour, then taste it again. How has the taste changed over the course of the hour? Why do you think this change occurred?
Observations and results
In this activity you should have observed a fizzing or bubbling when you added the baking soda to your lemon juice mixture. In addition, when you tasted your final product you should have also been able to feel the bubbles in your mouth—which were the product of an acid–base reaction. Can you guess which of the ingredients in your mixture was the acid and which was the base?
If you predicted the lemon juice was the acid, you're right! You can recognize acidic foods (such as lemons, which are very acidic) based on taste; acids taste very sour to us. Other acidic foods include vinegar, grapefruit and limes. Bases, on the other hand, can be more difficult to detect. Basic foods can taste slightly bitter to us—or they might have very little taste at all. In this case the base in this mixture was the baking soda, which doesn't have much of a taste. But you might have guessed it was the base in your reaction because as soon as you added it, your mixture should have started fizzing!
When the acid of the lemon juice (citric acid) came in contact with the carbonate base (baking soda) a chemical reaction took place, creating carbon dioxide gas (CO2). As you may know, CO2 is the same gas that is added to sodas to give them their fizz.
This reaction requires an acid and a base in order to take place. Therefore, when you tasted just the lemon juice or the lemon juice–water mixture you shouldn't have noticed any bubbles. Water is generally neutral (not an acid or a base)—thus, adding it to your lemon juice did not produce the reaction needed to create CO2 bubbles. It wasn't until you added the baking soda that the reaction took place, and the bubbles were created.
More to explore
Shimmy, Shimmy Soda Pop: Develop Your Own Soda Pop Recipe, from Science Buddies
Bath Bomb Science, from Science Buddies
Spurting Science: Erupting Diet Coke with Mentos, from Scientific American
Science Activities for All Ages!, from Science Buddies
This activity brought to you in partnership with Science Buddies

How To Draw Pop Fizz Step By Step
Source: https://www.scientificamerican.com/article/make-your-own-fizzy-lemonade/
Posted by: pepperhisday.blogspot.com

0 Response to "How To Draw Pop Fizz Step By Step"
Post a Comment